The SAE reconciliation process can be summarized in four - apparently easy - steps: retrieve and compare data, analyze the discrepancies, resolve the discrepancies and make the necessary adjustments in the clinical or safety database. But the process is more complex than it seems as it requires a thorough management of the numerous tasks involved, especially as all decisions and actions must be fully documented for GxP-compliance.
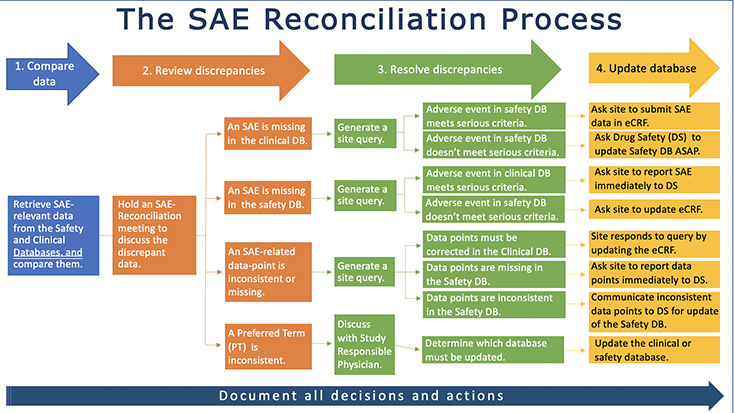
The SAE reconciliation process is driven by a Data Manager who, on a periodic basis, reviews data in the clinical database and compares defined variables with the corresponding records in the safety database. Verbatim descriptions, coding terms, dates and other information are frequently found to be different between the safety and clinical database and some SAEs maybe missing in one or the other database. Data Managers or CRO responsible Data Managers collaborate with Drug Safety for resolution.
An SAE-Reconciliation meeting is often necessary to discuss the discrepancies and determine if they are acceptable or decide on the appropriate course of action.
In most cases, queries are sent to sites for clarification or, in case of a Preferred Term (PT) inconsistency, the PT is discussed with the Study Responsible Physician or Safety Surveillance Physician.
Clinical monitors are in close contact with the investigational sites throughout the SAE reconciliation process and may be involved in query resolution and corrections. When the query response provides information that requires update of the safety database, a sponsor-specific GxP compliant process is followed for the update.
All decisions and actions must be documented. If certain data-points are not fully matching, the explanation for the discrepancy must be documented as well.
As a study progresses towards the end, the number of SAEs increases and the reconciliation activity becomes more intense. This is when the use of a GxP-compliant automated tool like Ethical eReconciliation offers competitive advantage by helping to save time while guaranteeing the quality and compliance of the SAE reconciliation process.
DOWNLOAD NOW THE FREE SAE RECONCILIATION HANDBOOK
The Manual / Reference Book with all the topics related to the Safety Data Reconciliation Management.







